








Copyright 2016 Biomedical Microdevices Laboratory
Current Research
Representative publications:
[1] O Khandan, A Famili, MY Kahook, and MP Rao. IEEE EMBC 2012.
[1] O Khandan, A Famili, MY Kahook, and MP Rao. IEEE EMBC 2012.
Ophthalmology
Research in this thrust focuses on addressing a critical unmet need, namely the development of a safe, simple, and efficacious means for delivering drugs to the eye. We have recently reported Ti-based microneedle (MN) devices that represent our first step towards achieving this goal [1].
MNs offer promise due to their diminutive size, which allows penetration into, but not through sclera or cornea. This provides a minimally- invasive means for precisely depositing drugs within such tissues, thus enabling circumvention of intrinsic transport barriers and clearance mechanisms, while also minimizing potential for retinal damage. Although both passive and active MNs have been reported, the former may prove more advantageous for eventual clinical use, due to their lower complexity and cost. However, potential for translation will be constrained by the limited carrying capacity of devices demonstrated to date, since this necessitates use of excessively large MN arrays to deliver required dosages.
The fenestrated Ti MNs shown here seek to address this limitation. Thus far, we have demonstrated that these devices: a) increase carrying capacity up to 5-fold relative to comparable solid MNs; b) provide potential for enhanced safety, due to their graceful, plasticity-based failure mode; and c) possess sufficient stiffness for corneal insertion.
Collaborators:
MY Kahook, Ophthalmology, University of Colorado School of Medicine
Research in this thrust focuses on addressing a critical unmet need, namely the development of a safe, simple, and efficacious means for delivering drugs to the eye. We have recently reported Ti-based microneedle (MN) devices that represent our first step towards achieving this goal [1].
MNs offer promise due to their diminutive size, which allows penetration into, but not through sclera or cornea. This provides a minimally- invasive means for precisely depositing drugs within such tissues, thus enabling circumvention of intrinsic transport barriers and clearance mechanisms, while also minimizing potential for retinal damage. Although both passive and active MNs have been reported, the former may prove more advantageous for eventual clinical use, due to their lower complexity and cost. However, potential for translation will be constrained by the limited carrying capacity of devices demonstrated to date, since this necessitates use of excessively large MN arrays to deliver required dosages.
The fenestrated Ti MNs shown here seek to address this limitation. Thus far, we have demonstrated that these devices: a) increase carrying capacity up to 5-fold relative to comparable solid MNs; b) provide potential for enhanced safety, due to their graceful, plasticity-based failure mode; and c) possess sufficient stiffness for corneal insertion.
Collaborators:
MY Kahook, Ophthalmology, University of Colorado School of Medicine
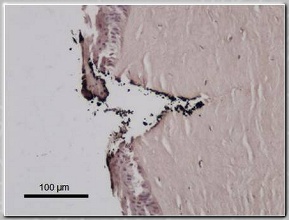
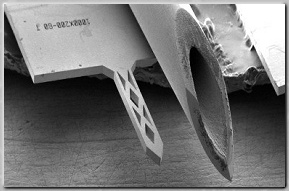
Top: Scanning electron micrograph of
fenestrated Ti microneedle and
conventional 26g stainless steel
hypodermic needle. Bottom: Histological
section of excised rabbit cornea at
insertion site of fenestrated Ti
microneedle (H&E stain) [1].
Sponsors:
UCR Regents Faculty Fellowship (PI: Rao)
UCR Regents Faculty Fellowship (PI: Rao)